PGTest
INTRODUCTION
PGTest is a type of Preimplantation Genetic Testing, screening for aneuploidy or monogenic disorder bearing embryos. This procedure ensures the optimal embryo selection process before implantation.
The selection of PGTest could screen for the optimal (in genetic) embryos. Thus, improve the implantation rate of IVF/ICSI cycles. In addition, PGTest helps significantly decrease the chance of having a child with an inherited genetic disorder or aneuploidy.
The PGTest tests are performed directly at GENTIS's laboratory by a team of leading experts in genetics and biotechnology, using the Veriseq kit and the new generation sequencing system by Illumina - USA and BlueFuse analysis software, contributes to providing genetic information of embryos, helps to select the best embryos for transfer and increases the chances of successful pregnancy.
WHO SHOULD TAKE PGTest
All couples undergoing in vitro fertilization (IVF) and couples with fertilized oocytes carry a high risk of genetic abnormalities such as:
- Women who are about to pregnant from over 35 years old;
- Couples with a history of miscarriage;
- Couples who have failed IVF;
- Male factors: consider low sperm count and/or quality defects.
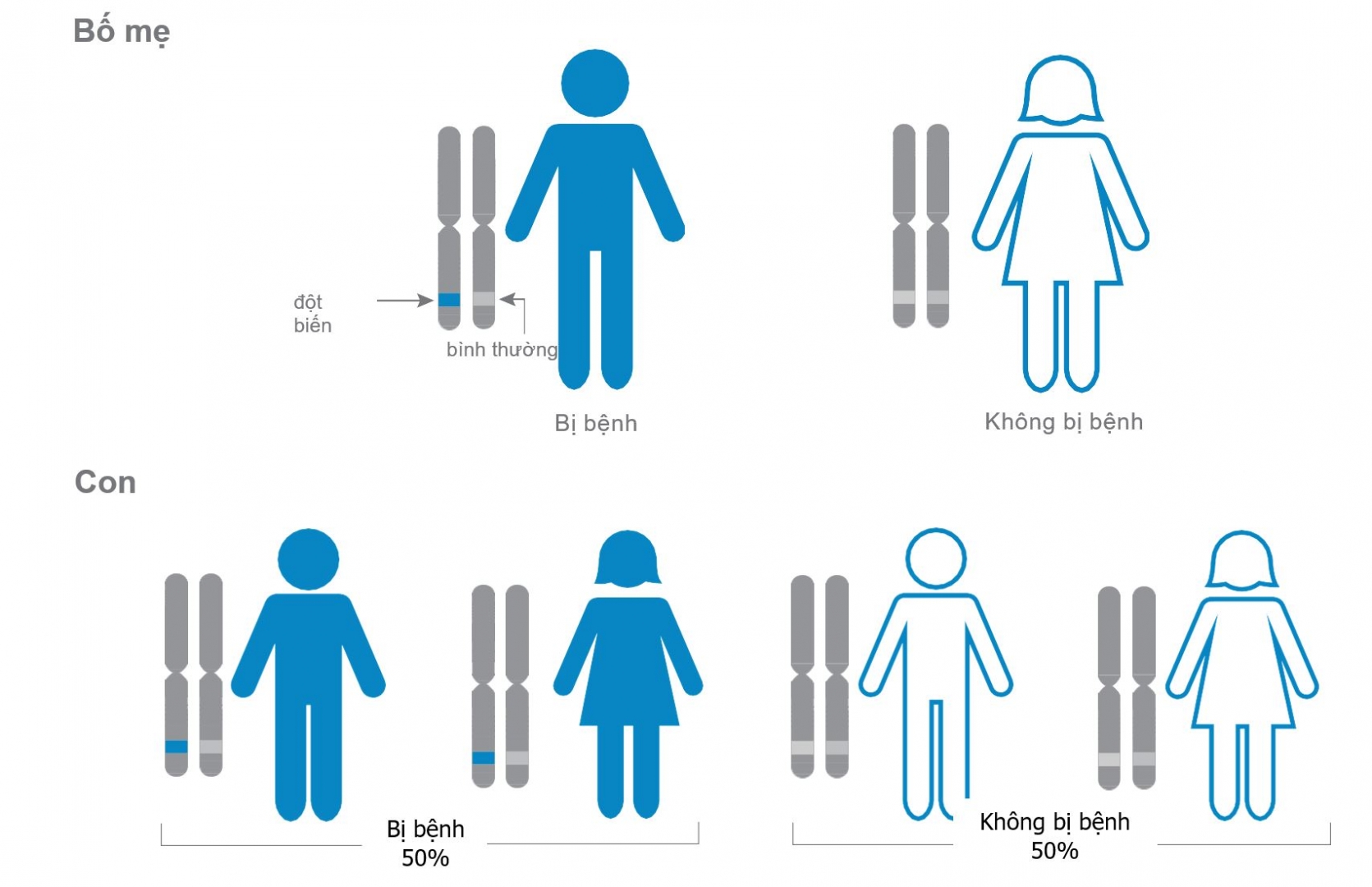
- Couples who have had children with genetic diseases, carriers of disease genes, chromosomal abnormalities, family history of genetic diseases.
Why should it be done?
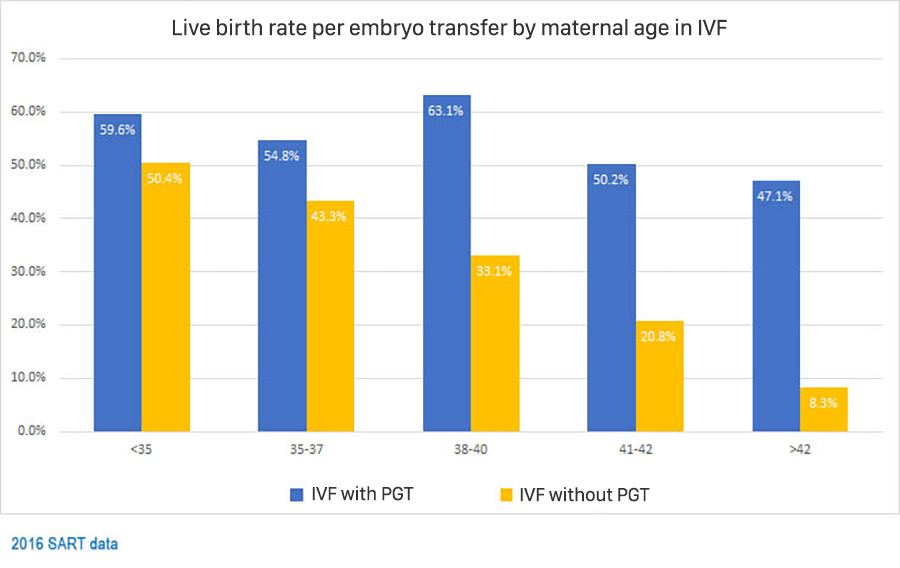
- Increase pregnancy rate per transfer: Selecting embryos that do not have abnormal chromosomes can increase pregnancy rates after transfer.
- Reduced miscarriage rate: In the general population, 25% of all clinical pregnancies end in miscarriage. The risk of miscarriage is reduced if embryos without abnormal numbers of chromosomes (euploid) are transferred.
- Reduce the risk of multiple pregnancies: Patients can confidently transfer one embryo that has been tested for chromosomes and does not have an abnormal number of chromosomes instead of transferring many untested embryos. Reduce the risk of multiple pregnancy.
- Increased chances of having a healthy baby: Some pregnancies with aneuploidy embryos can lead to an affected baby related to chromosomal syndromes (e.g. Down syndrome). .

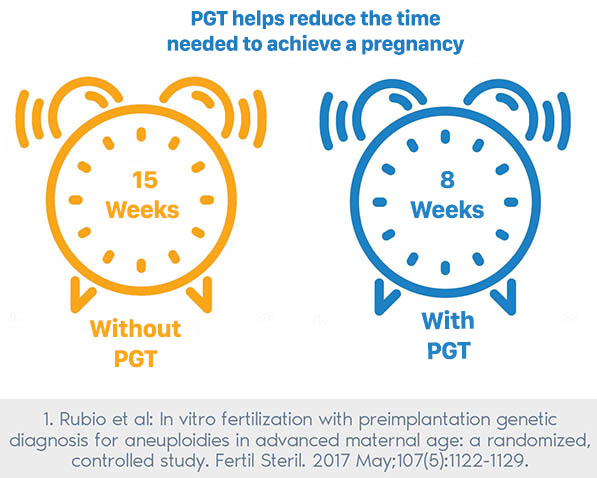
- Reduced time and resources needed: The time and resources required to achieve a pregnancy are reduced.
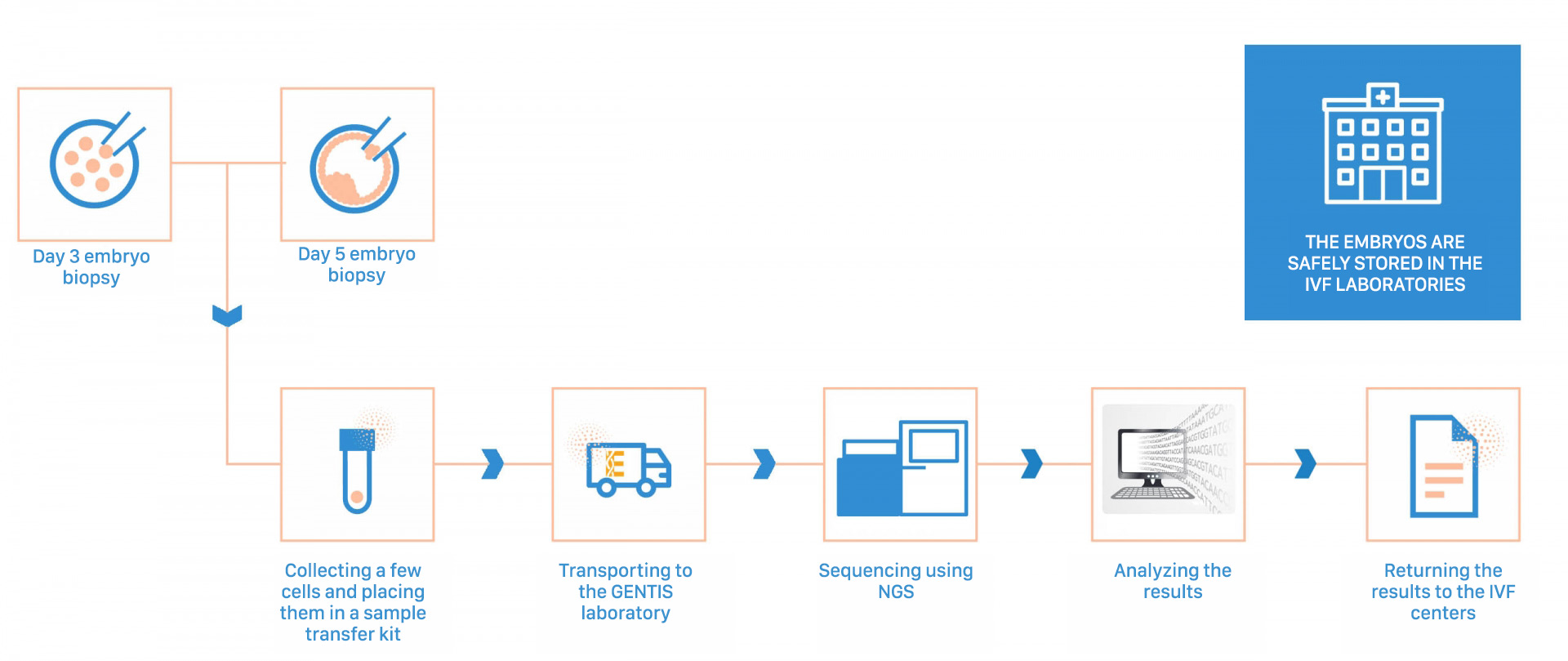
Testing procedure
PGTest at Gentis
GENTIS offers 3 types of PGTest:
- PGTest (PGT – A/SR)
- PGTest-M
- PGT Max 1
- PGTest - A/SR
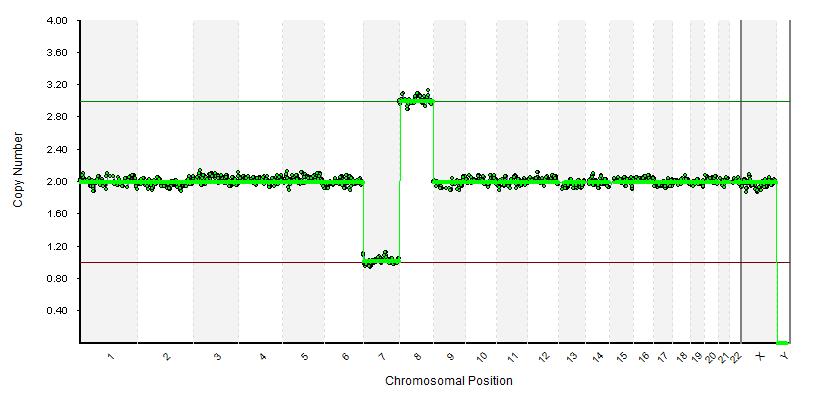
| DNA analysis detected aneuploidy of 24 pairs of chromosomes and structural abnormalities (PGT-A/SR) - Methods: Veriseq PGS – illumina, USA. High resolution with more than 3,000 data points; 100% sensitivity; Specificity 99.98%; Number of total reads >1,000,000/sample can detect deletions and insertions >5Mb in size - Technology used: New generation sequencing illumina - USA - Blue fuse analysis software can identify more than 130 syndromes associated with extra and deletion of chromosomes. - Samples used: Embryonic cells at the implantation stage on day 3-5 Note: No Reciprocal translocations detected |
- PGTest-M
| DNA analysis identifies single gene mutations (PGT-M): Associated with syndromes: Thalassemia, SMA, Hemophilia, RETT syndrome... - Methods: Analysis of SNP, STR to detect single gene mutations inherited from the father and mother - Technology used: CE-Thermo, NGS illumina – USA - Samples used: Embryonic cells in the implantation stage on day 3-5; Blood sample of father, mother or brother/sister carrying the disease (if any) |
- PGT Max 1
Advanced genetic analysis before embryo transfer, upgraded from the standard PGTest. This test detects common deletions as small as 2Mb, ensuring the birth of healthy children with the genetic abnormalities screened for.
PGTEST AT GENTIS
At GENTIS, we are committed to providing the most advanced PGT technology to enable the most accurate and informative selection of embryos.
- Veriseq PGS from illumina - USA
- Using the new generation DNA sequencing system of Illumina - USA.
- BlueFuse Multi result analysis software - USA.
- High resolution with more than 3,000 data points (probe) to help screen for abnormalities of all 24 chromosomes.
- Number of reads up to 1,000,000 reads/sample. Can detect loss and add fragments > 5Mb in size.
- 99.99% sensitivity; specificity 99.98%.
- Bluefuse analysis software identifies more than 130 syndromes associated with chromosome insertion and deletion.
Please fill in the information below to receive our supports and consultations!